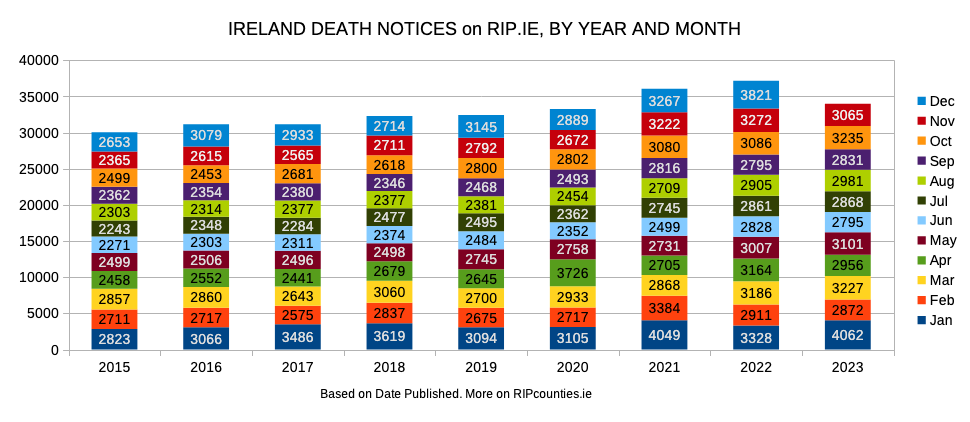
Today, Ireland Excess Deaths, Irish Quislings and RIP Counties each updated with their November numbers. Thankfully, this November seems less harsh than Nov ’22 and Nov ’21. But still way above pre-scam Novembers.
Also, more people have died in eleven months of 2023 than in the whole ‘novel virus’ year – the year we (supposedly) had no natural immunity nor injections to save us.
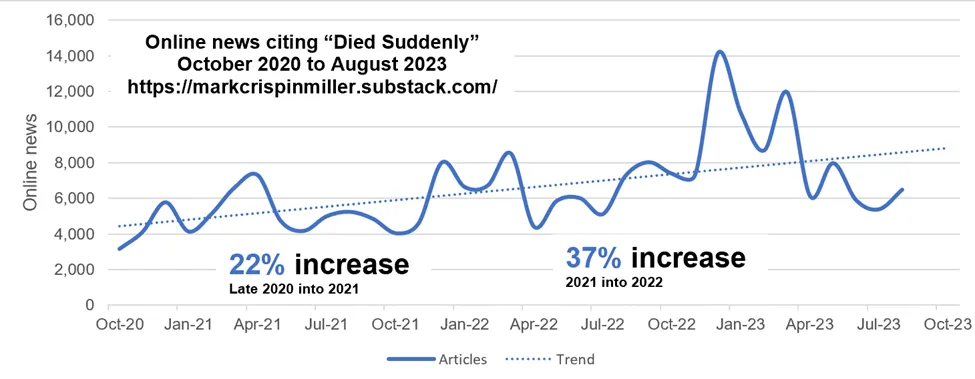
Good job those transfections started in Jan ’21 just as the deaths really started accelerating, eh?! It’d have been so much worse if those counter-measures hadn’t been deployed just then. Just in time.1Sarcasm
And no, this does not show lockdowns, masks and standing on circles in shops saved us in 2020. Because if they had saved us that year, why did they stop saving us from Jan ’21 on? (You might remember we were also doing those things for about the first half of 2021 – while the transfection roll-out was ramping up?).
- 1Sarcasm